
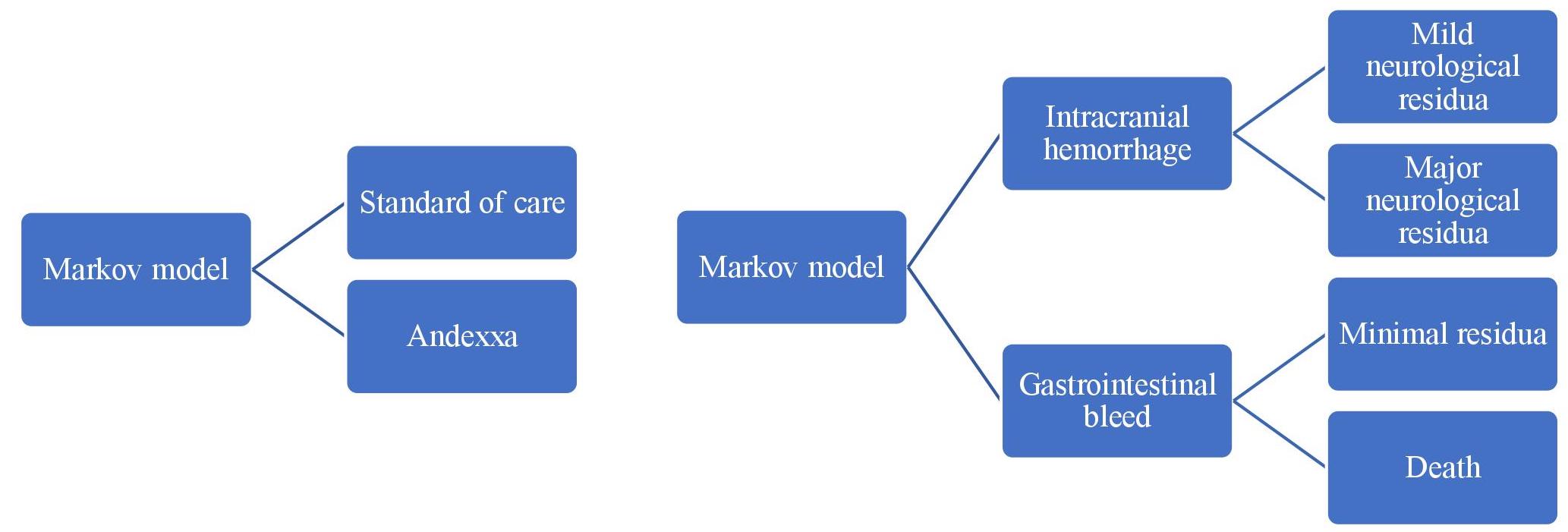
Lack of correlation between surrogate endpoint and clinical efficacy has also been well demonstrated in the field of neurocritical care.

Similarly, drugs reducing premature ventricular contractions, a marker for sudden cardiac death, actually increased mortality. This has been shown in clinical trials for oncology drugs when progression-free survival often does not correlate with overall survival. There is extensive literature showing that surrogate endpoints often do not correlate with improved patient outcomes. Superiority claims over PCC or stating Andexxa as standard of care seem unwarranted given the FDA’s assessment of clinical equipoise as well as the 59 centers listed as participating in the randomized control trial. Any committee deciding whether to add Andexxa to formulary should know the recommendation of the FDA clinical reviewers not to approve Andexxa whatsoever was overridden. Again, this implies PCC is the standard of care for non-vitamin K oral anticoagulant (NOAC)-induced severe bleeding. Approval for Andexxa may be withdrawn by the FDA if post-marketing studies fail to verify clinical benefit or not conducted with due diligence. Due to the magnitude of the uncertainty of the benefit, the FDA did mandate randomized clinical trial against standard of care which is currently PCC. However, the Director for the Office of Tissues and Advanced Therapies overruled the recommendation by the review team. FDA clinical reviewer and supervisors recommended against approval of Andexxa because they believe the “safety and efficacy data for ANDEXXA are not adequate to support approval”. They also had safety concerns given the in vitro effects and clinical thrombosis rates of up to 18% in early studies. The FDA clinical reviewers had concerns about the short half-life of Andexxa and the lack of correlation of in vitro activity with clinical efficacy. In this article, we discuss key aspects to consider when evaluating Andexxa for formulary addition. Furthermore, the off-label use and FDA concerns, conflicting society recommendations, and financial impact add another challenging layer to this evaluation. The ANNEXA-4 study and clinical trials with prothrombin complex concentrate (PCC) also have their limitations. Currently, there are no studies comparing the safety and efficacy of Andexxa to the current standard of care. The recent publication of ANEXXA-4 and the wider availability for Andexxa in 2019 has led many hospital Pharmacy and Therapeutics (P&T) committees to discuss whether Andexxa should be added to formulary. In May 2018, Andexxa (coagulation factor Xa, inactivated-zhzo or formerly andexanet alfa) received accelerated approval from the Food and Drug Administration (FDA) as a specific reversal for rivaroxaban- and apixaban-treated patients with life-threatening or uncontrolled bleeding despite not establishing improvement in hemostasis.


 0 kommentar(er)
0 kommentar(er)
